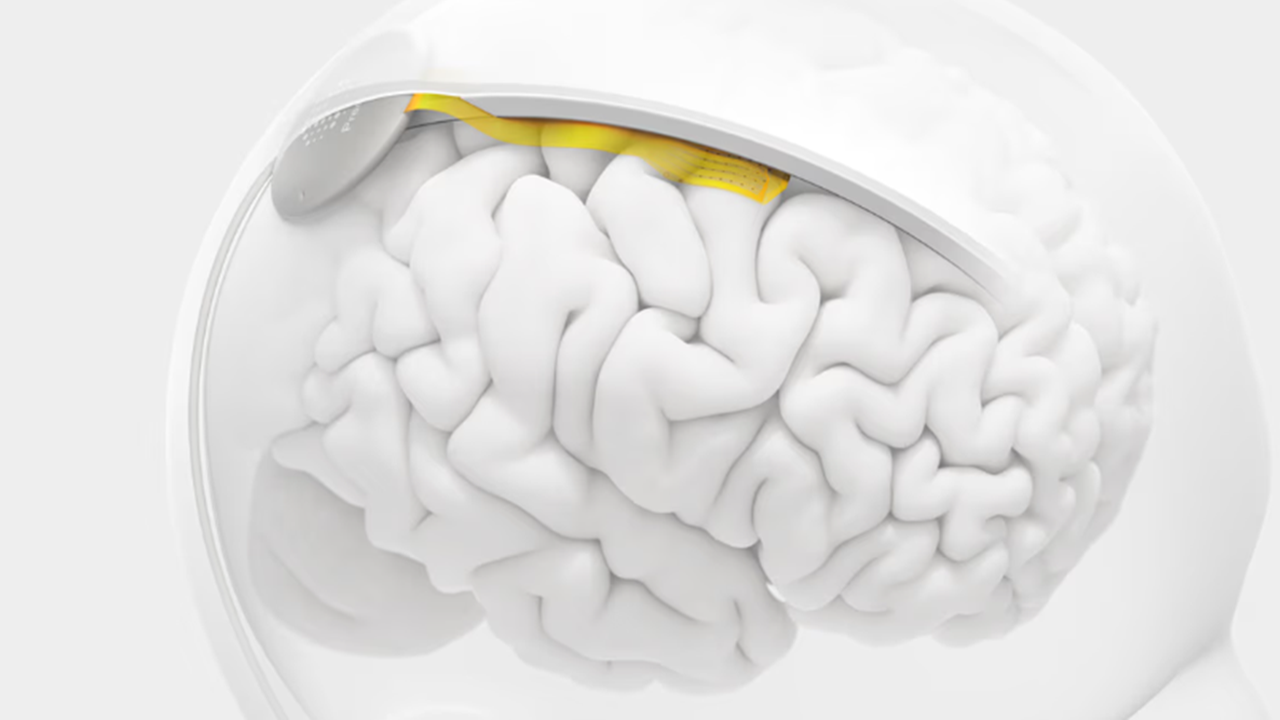
Layer 7 Cortical Interface Courtesy of Precision Neuroscience
Precision Neuroscience, a brain-computer interface (BCI) firm co-founded by former Neuralink co-founder Dr. Benjamin Rapoport, has received the FDA’s approval for its Layer 7 Cortical Interface.
The implant is made of microelectrode arrays embedded in a flexible, thin yellow film. Each array contains 1,024 electrodes thinner than a human hair. Multiple arrays can be combined to cover a larger area for data collection.
Precision’s Layer 7 Cortical Interface cleared for longer implantations.
When placed on the brain surface, it can seamlessly conform to the underlying tissue without penetrating or damaging it. A local processing unit is located between the scalp and the skull, which transmits the data to a connected digital device.
Precision has already implanted the chip in 37 patients for short periods, ranging from minutes to a few hours. The approval allows Precision to implant the chip for up to 30 days and collect more data.
“Neural decoding algorithms, like all AI-driven products, rely on vast amounts of data,” said Rapoport. “This regulatory clearance will exponentially increase our access to diverse, high-quality data, which will help us to build BCI systems that work more effectively.”
Precision is working on a unique wireless interface that will offer implant recipients more flexibility. It will be inserted anywhere on the brain surface through a sub-millimeter incision, which is totally reversible.
“We imagine a world where devastating neurological conditions—stroke, traumatic brain injury, dementia—are finally treatable,” said Precision CEO Michael Mager. “To reach this world, brain-computer interface technology needs to progress out of the lab and into the clinic.”
A single patient can receive multiple implants capable of recording information and simulating neural activity. It can also be used for other medical applications, such as brain mapping during open brain surgery.
The company is applying the technology for craniotomy procedures and epilepsy surgery at Beth Israel Deaconess Medical Center
“By introducing the Layer 7 Cortical Interface into clinical settings, we’ll be able to deliver immediate value for patients and neurosurgical teams, enabling real-time neural recording at a fidelity and scale not previously possible,” said Rapoport.
By December 2024, Precision had raised $107 million in venture capital funding from General Equity Holdings, B Capital, Steadview Capital, and Duquesne Family Office.
Precision Neuroscience faces stiff competition from Neuralink and Synchron.
While Precision is making steady progress in the brain-computer interface race, Elon Musk’s Neuralink has already implanted its BCI chip in three patients, achieving real-world application.
Precision also faces stiff competition from Australian startup Synchron, which is backed by Amazon’s Jeff Bezos and Microsoft’s Bill Gates.
In 2020, Synchron’s Stentrode Brain-Computer Interface received the FDA’s Breakthrough Device designation. Stentrode can be implanted in a blood vessel to simulate the nervous system without open brain surgery, making it cheaper, safer, and easier to implant than its competitors.
“As this is a first-of-its-kind device, we look forward to working closely with the FDA to prioritize development of the Stentrode and ensure access for patients with paralysis, as well as lay the groundwork for future indications for brain-computer interfaces,” said Thomas Oxley, MD, PhD, CEO of Synchron.
Meanwhile, driven by healthcare integration for neuro-rehabilitation and communication aids, the Brain-computer interface market is expected to reach $8.36 billion by 2032 with a 15.81% CAGR, according to research firm SNS Insider.




